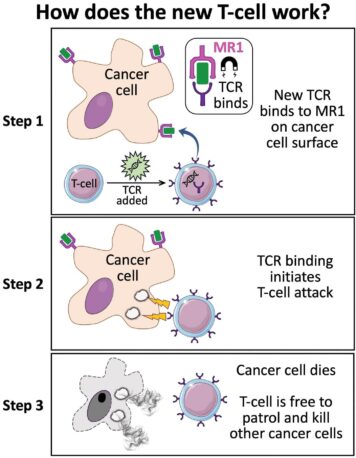
Early experiments show the new, as-yet-unnamed TCR can kill lung, skin, blood, colon, breast, bone, prostate, ovarian, kidney, and cervical cancer cells. A germ of all trades, if you will.
Lead study author Andrew Sewell, an expert in T-cells from Cardiff University, said it is “highly unusual” to find a TCR with such broad cancer specificity, raising the prospect of “universal” therapy.
“Current TCR-based therapies can only be used in a minority of patients with a minority of cancers,” he explained. “We hope this new TCR may provide us with a different route to target and destroy a wide range of cancers in all individuals.”
When injected into cancerous mice bearing a human immune system, the MR1-spotting T-cells showed “encouraging” cancer-clearing results.
The team also showed that modified T-cells of melanoma patients destroyed not only their cancer cells but those of other patients’ in the lab, regardless of HLA type.
“Cancer-targeting via MR1-restricted T-cells is an exciting new frontier,” Sewell said. “Previously nobody believed this could be possible.”
 English
English
 English
English
 English
English